

Come and have fun with
us!! Contact info: echampeil@jjay.cuny.edu
tel: 1 646 557 4502
Courses taught
 (1)
CHE 201, Organic chemistry I
(1)
CHE 201, Organic chemistry I
(2)
CHE 202, Organic Chemistry II
(3) CHE 201,
Organic Chemistry I, Hybrid course on line
(4) FOS 402 Undergraduate
Research Internship
Current
research interest:
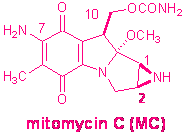
Mitomycin C chemistry
We are interested in the synthesis of mitomycin C-DNA
adducts. Mitomycin C ( MC1), an antitumor antibiotic, is used in
clinical cancer chemotherapy.2 Its cytotoxic and antitumor activity
is attributed to its ability to alkylate DNA monofunctionally and
bifunctionally, the latter mode resulting in DNA interstrand and intrastrand
cross-links3. Six major DNA adducts have been isolated from in vitro systems, formed under
biomimetic conditions, and their structures have been elucidated4,
including the DNA interstrand cross-link 3a
(ICL), the first such adduct of a natural antibiotic5 (see chart).
The same six DNA adducts were shown to form in tumor cells treated with MC6.
The ICL was also isolated from rat liver DNA of animals injected with the drug5.
The structures of the six MC-DNA adducts illustrate that the exclusive target
of alkylation of DNA by MC is the guanine base. Individual structure-activity
relationships of multiple DNA adducts generated by a single agent have been
investigated mostly in the case of organic mutagens and carcinogens7.
In general, such studies utilize synthetic oligonucleotides bearing a specific
adduct at a unique position of their base sequences. The MC adducts present an
opportunity for similar studies, enabling in this case direct comparisons of
biological effects of the various DNA adducts of a cancer chemotherapeutic
agent. Synthesis of most of the six MC adducts incorporated in oligonucleotides
has been accomplished by the biomimetic route, consisting of the alkylation
reaction of MC with a short DNA duplex of appropriate sequence in the presence
of a reductive MC-activating agent. Varying the activation conditions leads to
different adducts8. This approach has been successfully
applied in the case of most adducts 9,10,11. However, the method is usually inefficient,
due to the difficulty of purification of the product to homogeneity.
Furthermore, adducts 1b and 6 are formed in very low yield by the
biomimetic approach to be practical.
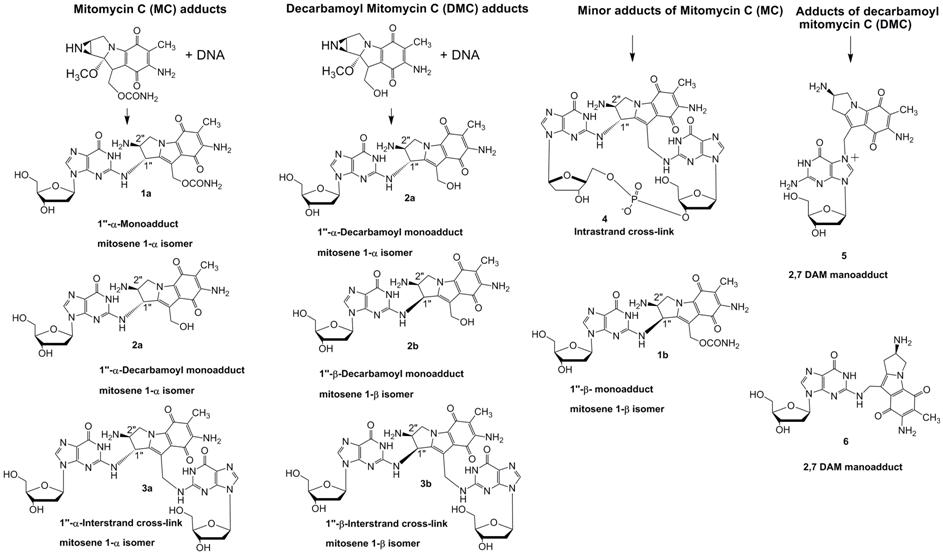
We reported the first alternative access to one of the
adducts of MC, based on organic synthetic methods, featuring a
postoligomerization approach12a,b in which the normal
nucleophile-electrophile relationship of the DNA nucleoside and the drug is
reversed by using an amine derivative of the latter and 2-fluorodeoxyinosine as
the DNA target site. Specifically, we described a synthesis of monoadduct 6 13 on both the nucleoside
and oligonucleotide levels. This synthetic approach to the previously
unavailable sister adduct 6 will
provide a substrate to examine the biological and structural properties of 6 in parallel with its major groove adduct counterpart 5. It will also be
interesting to compare properties of 6
with those of the other minor groove guanine-N2 adducts of MC, 1b
and 4. Our next target is to
find a synthetic route to the novel stereo-isomeric DNA adducts (beta adducts 2b and 3b)
that result from Decarbamoyl Mitomycin C treatment of cells.
It was found that DMC is
able to rapidly induce cell death in the absence of wild-type p53 protein
function and the markers seen during this cell death have features replication-stress induced death14,
the programmed necrosis pathway15, and may even be caused by
autophagy as this is an efficient p53-independent cell death pathway16.
One long-term goal of is to determine the p53-independent signaling pathway(s)
activated by DMC as a means to identify molecular targets for killing cancer
cells by non apoptotic death pathways.
In order to gain insight
into the damage signaling pathway, we want to determine the set of sensor
proteins which bind to the synthetic MC and DMC-adducts in the presence or
absence of p53. It has previously been shown that the damage induced by the two
drugs signals to different death pathways in the presence and absence of p53,
suggesting that the DNA-adducts serve as 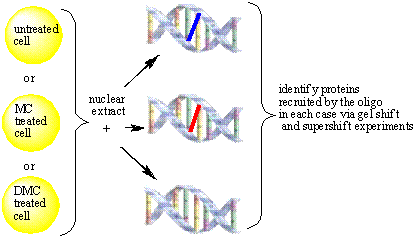 sensors.
sensors.
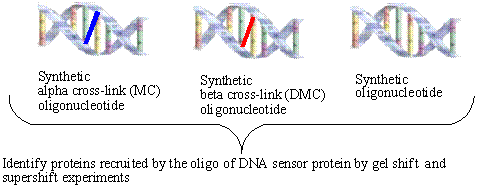
We also want to identify
which cellular proteins (from untreated cells, or MC or DMC treated cells) are
recruited by the synthetic lesion and non-lesion oligonucleotides. The nuclear
extract derived from the treated and untreated human cancer cells will be
compared for the ability of their proteins to bind to DNA by gel shift
experiments. A comparative analysis of
each nuclear extract with the specific adducts will allow us to determine if
specific factors have the capacity to bind to the altered stereochemistry and
if DNA damage is required to activate binding to the synthetic lesion. If
variable binding is determined for the different adducts, then experiments will
be carried out to determine the identities of the protein products.
(1)
Abbreviations: MC, mitomycin C; DAM, diaminomitosene; ICL,
interstrand cross-link.
(2)
Verveij, J. D. H.; Pinedo, H. M. In: Cancer Chemotherapy, Chabner, B. A.; Collins, J. M., Eds;
Lippincott; Philadelphia, PA, 1990; pp. 382-396.
(3)
Tomasz, M. Chem. Biol. 1995, 2, 575-579.
(4)
Palom, Y.; Belcourt, M. F.; Musser, S. M.; Sartorelli, A. C.;
Rockwell, S.; Tomasz, M. Chem. Res.
Toxicol. 2000, 13, 479-488.
(5)
Tomasz, M.; Lipman, R.; Chowdary, P.; Pawlak, J.; Verdine, G. L.;
Nakanishi, K. Science 1987, 235, 1204-1208.
(6)
Bizanek, R.; Chowdary, D.; Arai, H.; Kasai, M.; Hughes, C. S.;
Sartorelli A. C., Rockwell, S.; Tomasz, M. Cancer
Res. 1993, 53, 5127-5134.
(7)
Basu, A. K.; Essigmann, J. M. Mutation Res. 1990, 233, 189-201.
(8)
Tomasz, M.; Palom, Y. Pharmacol. Ther. 1997, 76, 73-87.
(9)
Kumar, S.; Lipman, R.;
Tomasz, M. Biochemistry 1992, 31, 1399-1407.
(10)
Borowy-Borowsky, H.;
Lipman, R.; Tomasz, M. Biochemistry 1990, 29, 2999-3006.
(11)
Suresh Kumar, G.; Musser, S. M.; Cummings, J.; Tomasz, M. J. Am. Chem. Soc. 1996, 118, 9209-9217.
(12)
a) DeCorte, B. L.;
Tsarouhtsis, D.; Kuchimanchi, S.; Cooper, M. D.; Horton, P.; Harris, C. M.;
Harris, T. M. Chem. Res. Toxicol. 1996, 9, 630-637. b) Cao, H.; Jiang, Y.; Wang, Y. J. Am. Chem. Soc. 2007, 129, 12123-1230.
(13)
Champeil, E., Paz, M.; Ladwa, S.; Clement, C.;
Zatorski, A.; Tomasz, M. J. Am. Chem. Soc., 2008, 130,
9556–9565.
(14)
Zhang, Y.W.; Otterness, D.M.; Chiang, G.G.; Xie, W.; Liu, Y.C.;
Mercurio, F.; Abraham, R.T. Molecular
cell 2005,19, 607-618.
(15)
Zong, W.X.; Thompson, C.B. Genes
& development 2006, 20, 1-15.
(16)
Nelson, D.A.; White, E. Genes
& development 2004, 18,
1223-1226.
Fullerene chemistry
Among promissing nanoparticles, fullerene C60 is
particularly interesting in the field of medicinal chemistry due to its hydrophobic nature and unique shape. In the context of a search of new fullerene-containing chemotherapeutic
agents, we already have synthesized a series of C60-derived building blocks
characterized by the presence of functional groups linked to the C60 backbone
by a flexible tether of variable
length. We selected organometallic reagents carrying orthoester, acetal and
silylether functional groups as precursors for carboxylic acids, aldehydes and
alcohols respectively1.
Our goal is to synthesize molecules which will
be more efficient chemotherapeutic agents than Mitomycin C. By linking MC to
fullerene C60, we want to increase the tumor cells targeting capability of MC
and we want to increase the cellular drug uptake and the DNA alkylation. We
strongly believe that the cellular drug uptake will be increased due to the
presence of the fullerenyl moiety as there is literature precedence of such a
phenomenon. Recently, Barron et al.
have described a general approach to the formation of a fullerene-containing
cell-penetrating peptide. By linking their fullerenyl amino-acid to a cationic
peptide, a prompt delivery of both the peptide and the fullerene components
inside the cell membrane was allowed whereas the peptide on its own could not
enter the cell2.
They concluded that the
fullerene moiety enabled the transport into cells of the peptide. Furthermore,
the authors noticed that these fullerenyl peptides were located in the nucleus
region of the cells. The fact that the cell uptake of their fullerenyl peptide
was found to be temperature dependant suggested that the cellular uptake
activity was an endocytosis process promoted by the hydrophobic nature of the
fullerene. They concluded that the hydrophobic fullerene in combination with
the hydrophilic peptide sequence may form an amphipatic cell penetrating
peptide.
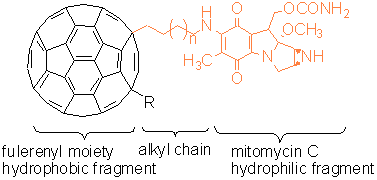
We want to synthesize molecules with a
fullerene-alkylchain-MC skeleton which will form amphipatic cell penetrating
structures. The hydrophobic moiety being the fullerene C60 and the hydrophilic
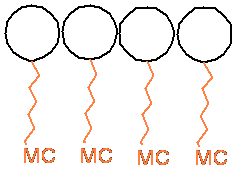
being the mytomicin structure. A cross section of the macro structures that can
be formed with our amphipatic molecules is represented below. The white sphere
represents the fullerene moiety, the orange fragment, the alkyl chain and the
mitomycin moiety.
(1) Champeil, E.; Crean, C.; Larraya, C.; Pescitelli, G.; Proni,
G.; Ghosez, L Tetrahedron 2008, 64,
10319–10330.
(2) a) Yang, J; Alemany, L. B.; Driver, J.; Hartgerink, J.
D.; Barron, A. R. Chem. Eur. J. 2007, 13, 2530-2545. b) Yang, J.;
Wang, K.; Driver, J.; Yang J.; Barron, A.R. Org. Biomol. Chem. 2007,
5, 260-266.
Drug of abuse characterization in
human fluids
Recently we started
to investigate the possibility of characterizing the presence of Drugs of abuse
in human fluids using NMR spectroscopy.
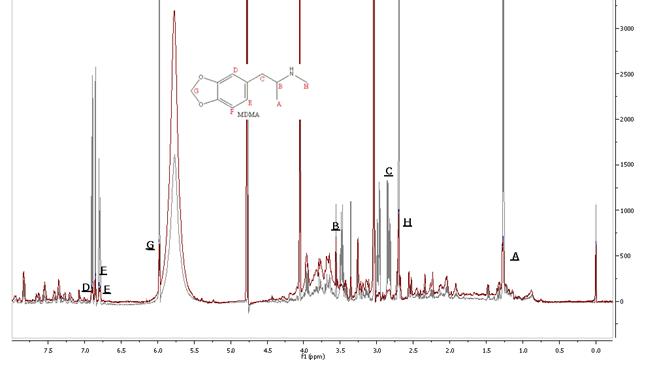
Below is a typical spectrum of a urine sample after ingestion of
3,4-methylenedioxy-N-methylamphetamine
(MDMA, ecstasy). Superimposed in gray is the spectrum of MDMA spiked urine
(0.50 mg/mL). Characteristic peaks of the drugs are clearly visible. These results
suggest the 1H NMR spectroscopy could provide a convenient tool for the rapid
detection of MDMA in human urine.
This method
presents the advantage of a rapid diagnosis with little of urine needed and no
sample preparation. Furthermore, in the concentration range studied,
quantitative data can be collected and samples were analyzed within 20-30
minutes. In an emergency clinical context, the diagnosis problem could be at
least partially solved if a rapid identification procedure was available. The
NMR method should be useful in rapidly confirming the diagnosis of poisoning.
Of course,
all this would not be possible without the contribution of these wonderful John
Jay Students:
Elaan
Luckaziewitcz, Kyle Zavinsky, Samantha Sellers, Stephanie Watson, Sandy Kong,
Jonathan Liu, Casey Lesar
![ads dinner 014[1].JPG](index_files/image031.jpg)
Selected
Recent Publications
●Elise Champeil,
Gloria Proni, Danielle Sapse, “Ab Initio studies of receptor interactions with
AMPA ((S)-2-amino-3-(3-hydroxy-5-methyl-4-isoxazolyl)
propionic acid ) and kainic acid (2S-(2α,3β,4β))-2-carboxy-4-(1-methylethenyl)-3-pyrrolidineacetic
acid”, Journal of Molecular Modeling 15 (2009): 1109.
●Elise Champeil, Conor Crean, Carlos Larraya, Gennaro
Pescitelli, Gloria Proni , Léon Ghosez, “Functionalization of C60 via
organometallic reagents”, Tetrahedron
64 (2008): 10319.
●Manuel M. Paz, Sweta Ladwa, Elise
Champeil, Li-Quian Tang, Sara Rockwell, Ernest Boamah, Jill
Bargonetti-Chavarria, John Callahan, John Roach, Maria Tomasz, “Mapping DNA
adducts of mitomycin C and decarbamoyl mitomycin C in cell lines using liquid
chromatography/ electrospray tandem mass spectrometry”, Chemical Research in Toxicology 21 (2008): 2370.
● Danielle
S. Sapse, Elise Champeil, Jacques Maddaluno, Catherine Fressigné,
Anne-Marie Sapse, “Ab initio study of the interaction of DNA fragments with
methyllithium”, Compte rendu des Séances
de l’Académie Francaise 11
(2008): 1262.
● Elise Champeil, Manuel Paz, Sweta Ladwa, Cristina Clement C, Andrzej Zatorski
, Maria Tomasz, “Synthesis of an
oligodeoxyribonucleotide adduct of mitomycin C by the postoligomerization
method via a triamino mitosene”, Journal
of American Society 130 (2008): 9556.
● Elise
Champeil, Padmanava
Pradhan, Mahesh K. Lakshman, “Palladium-catalyzed synthesis of
nucleoside adducts from bay and fjord region diol epoxides”, Journal
of Organic Chemistry 72 (2007): 5035.